Introduction
A retrospective study (Russell JA et al. BBMT 2010) showed additional 400 cGy of total body irradiation (TBI) to Flu/Bu regimen improved both overall survival and disease-free survival. To investigate the role of additional TBI 400 cGy in Flu/Bu4 conditioning regimen, we conducted a prospective randomized comparative study of fludarabine/busulfan (Flu/Bu4) and fludarabine/busulfan/total body irradiation (Flu/Bu4/TBI) conditioning regimen in allogeneic hematopoietic stem cell transplantation for patients with myeloid diseases.
Methods
We prospectively enrolled patients with myeloid diseases and randomized to Flu/Bu4 or Flu/Bu4/TBI between July 2010 and August 2019. Patients were stratified by diagnosis and disease risk. Patients received fludarabine 40 mg/m2 on days -6 to -3 followed by IV busulfan 130 mg/m2 on days -6 to -3 (Flu/Bu4). Flu/Bu4/TBI group had additional 400 cGy TBI in two fractions on day -1. Recipients had rATG 4 mg total dose over 3 days on days -3 to -1. All patients received post-transplant immunosuppression with tacrolimus and methotrexate 5 mg/m2 on days +1, 3, 6, and 11. We analyzed overall survival (OS), leukemia free survival (LFS), non-relapse mortality (NRM), relapse, acute- and chronic graft versus-host disease (GvHD).
Results
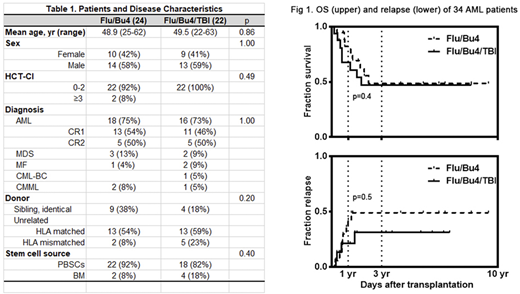
Forty-six (27 male and 19 female) patients with myeloid diseases were randomized into Flu/Bu4 (24 patients) or Flu/Bu4/TBI (22 patients). Patients had AML (34 patients), CML (1 patient), MDS (5 patients), MF (3 patients), and CMML (3 patients) with mean age 49.2 (22-63 years) (Table 1). There were no graft failures.
One patient died within 100 days of transplantation due to organ failure. Among 46 patients, eleven patients died within a year of transplantation (5 patients in Flu/Bu4, 6 patients in Flu/Bu4/TBI), 5 from disease progression, 3 from acute GVHD, 1 from infection, 1 from fall, and 1 from organ failure.
Day 100 grade II-IV and III-IV acute GVHD rates were 45.7% (95% CI [31.3, 60.1]) and 10.9% (95% CI [1.9, 30.8]), respectively; 1-year chronic GVHD and extensive chronic GVHD rates were 39.1% (95% CI [25.0, 53.2]) and 13.0% (95% CI [3.3, 22.8]), respectively.
With a median follow up of 22.6 months (range, 1.8-114.7), NRM at day 100 was 2.2% (n=1) and 10.9% at 1 year. Overall survival (OS), leukemia-free survival (LFS) and relapse at 1-year were 74.4%, 41.9%, and 30.2%, respectively.
Among 34 AML patients, LFS for all AML patients was 52.9% at 1 years. LFS was higher in patients undergoing Flu/Bu4/TBI with 56.3% versus Flu/Bu4 with 50.0% at 1 year (p=0.4). AML relapse with Flu/Bu4 and Flu/Bu4/TBI regimen was 38.9% versus 18.8% in the first year, 44.4% versus 25.0% in three years (RR=0.61 95% CI [0.20, 1.91]) (Fig 1). However, Flu/Bu4/TBI regimen increased the risk of NRM in AML patients (60.0% versus 14.3%, p=0.22).
Overall survival (OS) for all AML patients was 67.6% at 1 year. OS of AML patients was similar in patients undergoing Flu/Bu4 with 72.2% versus Flu/Bu4/TBI with 62.5% at 1 year (p=0.4). The median survival was 26.9 months and 21.4 months, respectively.
Conclusions
Addition of TBI 400 cGy to Flu/Bu4 conditioning regimen resulted in reduction of relapse, contributing to a better LFS. However, Flu/Bu4/TBI group did not have a better OS due to the regimen related mortality. These findings suggest the additional TBI 400 cGy to Flu/Bu4 should be cautiously selected for patients who is fit but has a high risk of relapsed disease.
Rowley:FATE Therapeutics: Consultancy; AbbVie: Current equity holder in publicly-traded company. Skarbnik:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CLL Society: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Verastem: Speakers Bureau; Beigene: Speakers Bureau; Alexion: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vesole:Janssen: Speakers Bureau; Sanofi: Speakers Bureau; BMS: Speakers Bureau; Amgen: Speakers Bureau; Takeda: Speakers Bureau. Donato:Genomics Cooperative: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.